New advancements in energy storage are set to propel batteries used in electric aviation and grid power.
A research team headed by Chinese researcher Wang Chunsheng, a professor in the Department of Chemical and Biomolecular Engineering at the University of Maryland, has made significant advancements in the field of aqueous battery electrolytes....
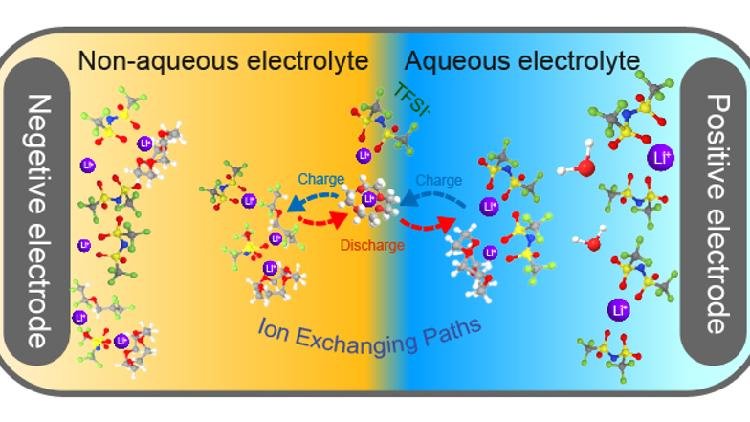
Published in the journal Nature Nanotechnology on April 8, this innovation is anticipated to connect the current commercial aqueous batteries, such as lead-acid and nickel-metal hydride, with advanced non-aqueous lithium-ion batteries.
"We developed membrane-free aqueous/organic bi-layer electrolytes and reduced interface resistance and mixing between the aqueous and organic phases by adding super-lithophilic ionophores," stated Dr. Zhang Xiyue, the first author of the study.
Water-based electrolytes are attracting global attention due to their inherent safety and environmentally friendly characteristics, making them promising candidates for next-generation energy storage solutions. Nonetheless, a significant hurdle has remained: the narrow electrochemical stability window, which limits the working voltage of aqueous batteries and thereby constrains their energy density and application range. Wang's previous research introduced a water-in-salt electrolyte in 2015, boosting the stability window from 1.23V to 3.0V, although some challenges persisted.
Despite the 3.0V stability window of water-in-salt aqueous electrolytes, compatibility issues with high-energy lithium metal or graphite anodes—critical components for high-energy batteries—have been a barrier. This voltage mismatch has created a bottleneck in advancing aqueous batteries to higher energy densities.
To address this issue, Wang and postdoctoral researcher Zhang Xiyue at UMD devised a new electrolyte system capable of functioning at an unprecedented voltage range of 0.0-4.9V. This development breaks the long-standing reduction potential limit of aqueous electrolytes, extending it from 1.3V down to 0.0V, thereby paving the way for genuinely high-energy-density aqueous batteries.
The testing phase revealed that the team's model battery, utilizing this new electrolyte system, maintained stable performance after over 2,000 cycles, showcasing remarkable long-term durability.
This technology presents exciting possibilities across various applications, such as electric aviation, large-scale low-carbon grid storage, and even lithium extraction from seawater.
Acknowledging the crucial role of solvent structure regulation in enhancing battery performance, Wang and Zhang have also published a detailed review in the journal Advanced Materials. This article discusses the fundamental principles and vital metrics for aqueous electrolyte design, evaluates current scientific challenges, and suggests innovative strategies for the future.
Collectively, these findings advance the boundaries of aqueous electrolyte development, establishing a theoretical and technological basis for the creation of next-generation energy storage systems that are both safe and highly efficient.
Rohan Mehta for TROIB News
Discover more Science and Technology news updates in TROIB Sci-Tech












